
- Services
- CMC total service
Total CMC Services by Expert Biologists
Fast, Efficient, High-Quality Services
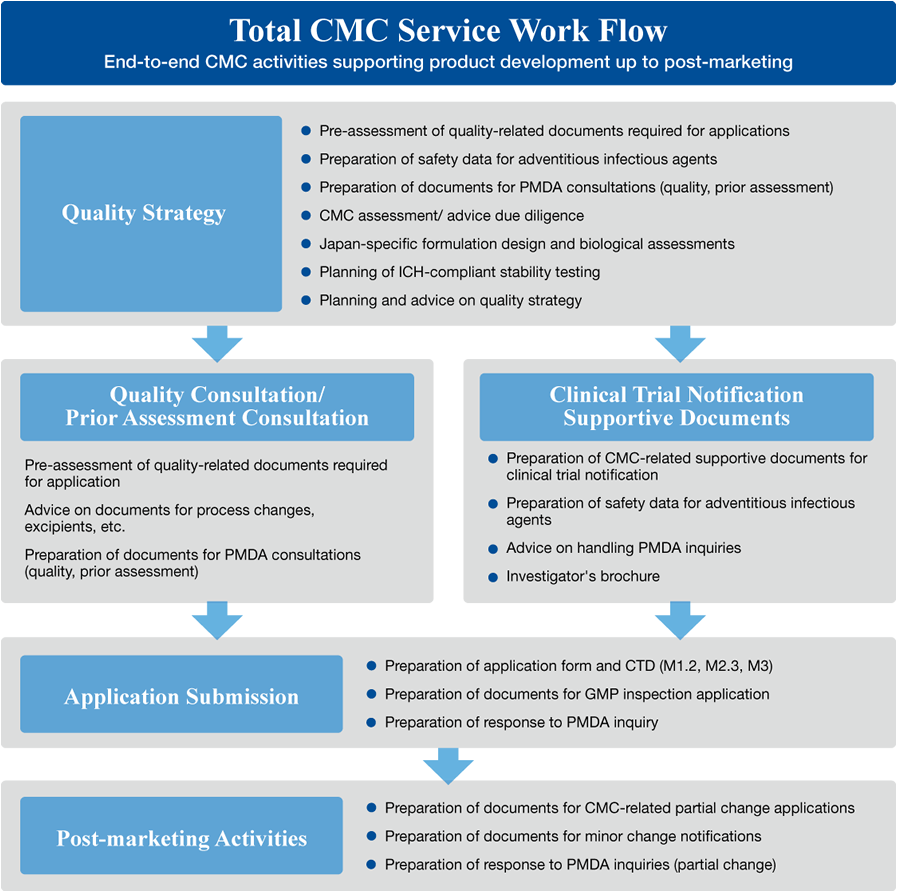
Business content
-
Document Preparation
- Drug Quality Consultations; Ad Hoc Consultations
- Pre-Assessment Quality Consultations
- Pre-Phase 1 study Quality-related Consultations
- Supporting Documents for Clinical Trial Notification
- JAN Applications
- Foreign Manufacturer Accreditations
- GMP Inspection Applications
- New Drug Application Forms and CTD
- Master File Registration Applications
-
Consulting
- Quality-related Regulatory strategy planning
- CMC assessment advice / due diligence
- GMP compliance support
- Advice on quality consultation with authority
-
Translation
- Translation service by specialists in biology/CMC
- Support on CMC-related communications with overseas clients’ headquarters
Our Activities
- We provide CMC services in the areas of biological drugs (antibody drugs, vaccines, recombinant products, biosimilars), synthetic drugs, and generics,among others.







